Below is a graph of the ionization energies for a particular element. 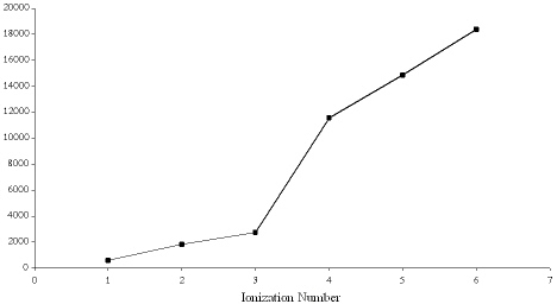 Based on the trend in the ionization energies the element is most likely
Based on the trend in the ionization energies the element is most likely
A) Ne
B) B
C) S
D) Al
E) K
Correct Answer:
Verified
Q1: Before the discovery of the element
Q2: The Group II alkaline earth metals for
Q3: The elements in Group VI, the chalcogens,
Q4: Which of the following has the lowest
Q5: Which is the largest energy?
A) The first
Q7: Arrange the following in order of increasing
Q8: Which of the following statements best describes
Q9: Ba2+ has
A) 0 valence electrons and 54
Q10: Given the following the data, what
Q11: Based on Coulombic forces, which would you
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents