In the Lewis diagram for SCN-, the formal charges are 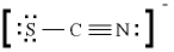
A) S (-1) , C (0) , N (+1)
B) S (-1) , C (0) , N (0)
C) S (0) , C (-1) , N (0)
D) S (-1) , C (+1) , N (-1)
E) S (+1) , C (0) , N (-1)
Correct Answer:
Verified
Q15: Use electronegativity difference to arrange the following
Q16: HCl has a bond length of 1.284
Q17: Which of the following is the best
Q18: Choose the best statement regarding why diagram
Q19: Draw a Lewis diagram for HCN to
Q21: Below are two equivalent resonance structures for
Q22: When drawing the Lewis diagram phosphine, PH3,
Q23: Using VSEPR theory, give the steric number
Q24: Using VSEPR theory including any distortions from
Q25: Which will have the smallest angle between
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents