Which of the following best describes the relationship between the atoms described below?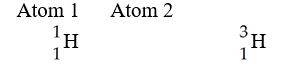
A) They are isomers.
B) They are polymers.
C) They are isotopes.
D) They contain 1 and 3 protons, respectively.
E) They each contain 1 neutron.
Correct Answer:
Verified
Q1: Molybdenum has an atomic number of 42.
Q2: Why is each element unique and different
Q3: Which of the following statements is false?
A)
Q5: Atoms whose outer electron shells contain 8
Q9: The precise weight of a mole of
Q10: Carbon-12 is the most common isotope of
Q11: Electrons exist only at fixed levels of
Q12: One difference between carbon-12 ( 
Q15: Oxygen has an atomic number of 8
Q20: About 25 of the 92 natural elements
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents