According to the arrangement of elements in the periodic table, which of the following elements would be expected to have chemical properties most similar to those of nitrogen, N, whose atomic number is 7?
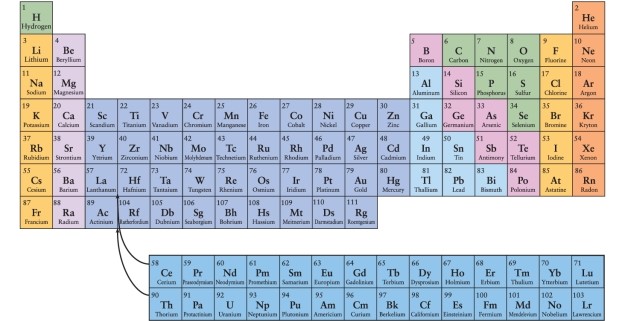
A) oxygen (O, atomic number 8)
B) phosphorous (P, atomic number 15)
C) chlorine (Cl, atomic number 17)
D) carbon (C, atomic number 6)
Correct Answer:
Verified
Q62: You are looking at a glowing field
Q63: The overall diameter of a typical atom
Q64: The typical size of an atom is:
A)10-6
Q65: The mass of a proton is:
A)about twice
Q66: The majority of the naturally occurring elements
Q68: Isotopes of a particular element in the
Q69: How many neutrons are there in the
Q70: How do the spectra of the two
Q71: One atom of 13C has how many
Q72: The specific sequence of spectral line series
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents