Match the definition with the quantity it is describing.
-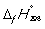
A) Energy required to melt one mol of a pure substance at its melting point, Tm, at 1 bar pressure
B) Energy required to vaporize one mole of a pure liquid at its boiling point, Tb, at 1 bar pressure
C) Enthalpy change at 298 K when 1 mol of a compound is formed under standard conditions from its constituent elements in their standard states
D) Enthalpy change when 1 mol of a substance reacts completely with oxygen gas at 1 bar pressure
Correct Answer:
Verified
Q13: The specific heat capacity of liquid water
Q14: The specific heat capacity of Cu (s)
Q15: The specific heat capacity of iron (Fe(s))
Q16: Match the definition with the quantity
Q17: Match the definition with the quantity
Q19: Match the definition with the quantity
Q20: The standard enthalpy change of formation is
Q21: The standard enthalpy change of fusion and
Q22: The standard enthalpy change of vaporization of
Q23: Calculate the enthalpy change (in kJ
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents