-tungsten crystallises with a body centred cubic structure where the length of the unit cell is 3.150 Å. Use the diagram to calculate the radius of a tungsten atom. (Hint: The radius of the atom is just half the distance between the two closest atoms.) 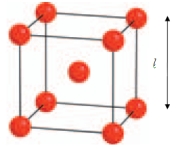
A) 2.692 Å
B) 1.136 Å
C) 1.346 Å
D) 1.575 Å
Correct Answer:
Verified
Q8: The structure of C60 is analogous to
Q9: Silicon dioxide exists as different polymorphs e.g.
Q10: A magnetic levitation train uses the Meissner
Q11: The structure with repeat sequence ABCCABCCABCCABC is
Q12: A body centred cubic unit cell is
Q14: A lattice point on the edge of
Q15: A semiconductor has which of the following
Q16: What is the formula of a compound
Q17: Li2O crystallises with the antifluorite structure where
Q18: Lattice point counting for sphalerite shows that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents