In the figure below, the shape of the curve is dictated by which of the following factors? Please select all that apply.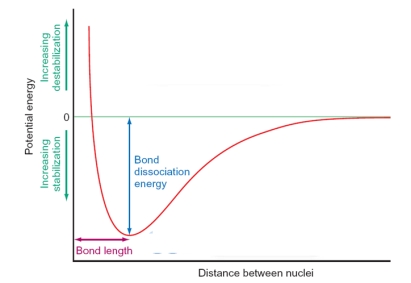
A) At long distances there is no interaction between the atoms as the orbitals are not able to overlap.
B) At very short distances the atoms repel strongly as the positive nuclei come into close contact.
C) The equilibrium between repulsive and attractive forces generates the bond length between the atoms.
D) As the atoms become close enough together the orbitals overlap and the atoms are drawn together as bonding occurs.
Correct Answer:
Verified
Q1: How do the bond enthalpies and bond
Q2: For a homonuclear diatomic, the bond length
Q4: The Lewis model can be used to
Q5: According to Lewis theory, a nitrogen atom
Q6: The dot and cross diagram for ammonia
Q7: The bonding of which of the following
Q8: _ species contain unpaired electrons and are
Q9: The electronegativity of an s or p
Q10: Interhalogen compounds of the form XF exist
Q11: Valence bond theory can be used to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents