Under what conditions would the reaction be most likely to proceed to the product shown at a reasonable rate? (Select one.) 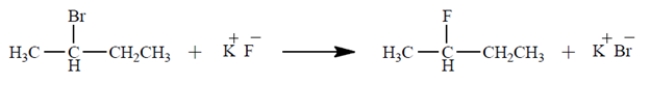
A) in a hydrocarbon solvent such as hexane
B) in ethanol solvent
C) in an ethanol solvent containing some water
D) in very dry dimethyl sulfoxide (DMSO, a polar aprotic solvent) containing a K+-bonding crown ether
E) in dimethyl sulfoxide containing a trace of water and a K+-bonding crown etherf.
in water containing enough acetone to dissolve the alkyl halide, plus a K+-bonding crown ether
Correct Answer:
Verified
Q6: Complete the SN2 reaction on (R)-2-iodobutane and
Q7: Rank the compounds in order of greatest
Q8: What are the two products that form
Q9: Write the mechanism of the reaction (that
Q10: Give the structure of the nucleophile that
Q12: Which compound will undergo the fastest SN1/E1
Q13: Write the two possible
Q14: Predict the organic product(s) of the reaction
Q15: According to the rate law for the
Q16: When we say, "sodium ethoxide (Na+ EtO−)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents