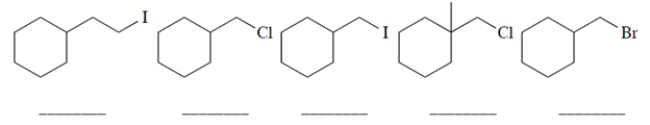
Rank the starting materials in terms of their rate of product formation in a reaction with sodium fluoride in dimethylformamide (1 being the fastest and 5 being the slowest). (Dimethylformamide (DMF) is a polar aprotic solvent.)
Correct Answer:
Verified
Q15: According to the rate law for the
Q16: When we say, "sodium ethoxide (Na+ EtO−)
Q17: Which compound will be most reactive in
Q18: Consider this reaction: Q19: Which species would act as the best Q20: Give the two organic products of the Q21: Which compound undergoes the fastest SN2 reaction Q22: Which compound undergoes the fastest SN1 solvolysis Q23: Which of these compounds will undergo the Q25: Rank these starting materials on their rate
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents