Consider the equilibria in CCl4, an apolar, aprotic, nondonor solvent.
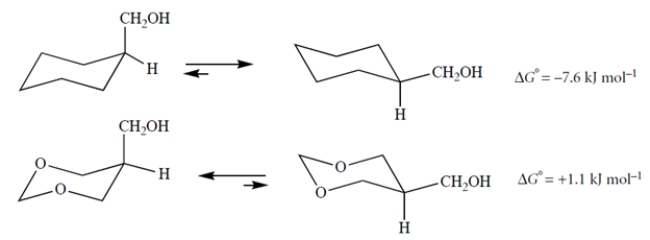 Explain why replacing the two carbons of the ring shown above with oxygens makes the axial conformation more stable.
Explain why replacing the two carbons of the ring shown above with oxygens makes the axial conformation more stable.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Draw the structure of 3-hexyn-2-ol.
Q16: Name the compound. Include the appropriate stereochemical
Q17: Name the compound. Include the appropriate stereochemical
Q18: Draw the structure of 3-ethoxy-2-butanethiol (alternate name
Q19: Given four compounds with the same branching
Q20: Which structure has the lowest melting
Q22: Compounds consisting of molecules that can both
Q23: Drugs are often conjugated to glucuronic acid
Q24: This is the predominant form of the
Q25: Draw all the alcohols having a molecular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents