Consider the reaction, in which X is a stable, mercury-containing compound (not a reactive intermediate). Compound Y does not react with either Br2, H2/catalyst, or ozone.
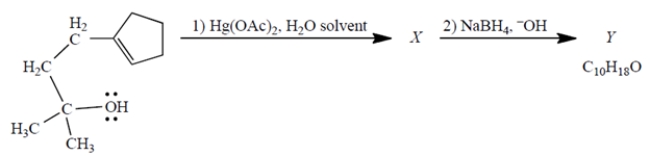 a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
b. Use mechanistic reasoning to deduce the structure of X. Use the curved-arrow notation; show all relevant unshared pairs and charges. Then, using what you know about step (2)-no mechanisms!-deduce the structure of the product Y.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q12: Identify the reagents needed to transform isopentene
Q13: A student allowed an alcohol, 4-penten-1-ol, to
Q14: Complete the reaction by providing the structure
Q15: Complete the reaction by providing the structure
Q16: Complete the reaction by providing the structure
Q18: Complete the reaction by providing a starting
Q19: Draw the structures of three enols that
Q20: Complete the reaction by providing the structure
Q21: Complete the reaction by providing the structure
Q22: Compare the results of hydroboration-oxidation and Hg2+-catalyzed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents