Consider the reaction free-energy diagram for two reactions that convert reactants (R) into products (P) and select the two correct statements.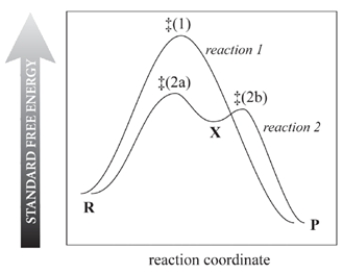
A) Reaction 1 is faster because it occurs in one step.
B) Reaction 2 is faster than reaction 1.
C) Both reactions occur at the same rate at a given concentration of R.
D) X is a transition state of reaction 2.
E) The rate-limiting step of reaction 2 is R ⇌ X.f.
The rate-limiting step of reaction 2 is X ⇌ P.g.
X must be a carbocation.
Correct Answer:
Verified
Q5: An alcohol has an empirical formula of
Q6: Give the structure of the HBr addition
Q7: Name the compound. Include an E or
Q8: Consider this molecule: Q9: Provide an IUPAC name for the compound. Q11: Give the IUPAC name, including stereochemistry, of Q12: Give the structure of the carbocation intermediate Q13: Name this compound (including stereochemical configuration where Q14: 14. In each pair, identify the more Q15: 14. In each pair, identify the more![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents