A multistep reaction A ⇌ B ⇌ C ⇌ D has the free energy-reaction coordinate diagram shown below. Which step of the reaction is rate-limiting in the forward direction?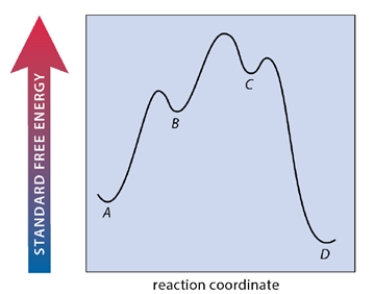
A) the reaction of A to B
B) the reaction of B to C
C) the reaction of C to D
Correct Answer:
Verified
Q16: In this pair of compounds, identify the
Q17: Which of these alkenes has the smallest
Q18: Complete the reaction by giving the major
Q19: Complete the reaction by giving the major
Q20: Complete the reactions by giving the major
Q21: Select the true statements that apply to
Q22: Complete the reaction by giving the structure
Q23: Provide an IUPAC name for the following
Q25: This carbocation undergoes rearrangement. Give the structure
Q26: Identify the most stable carbocation:![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents