The amino acid arginine (Arg, R) exists at pH = 7.4 as the following structure:
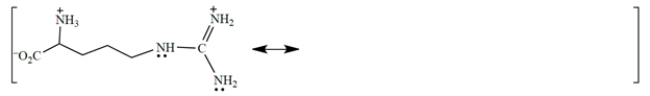 a. The cation in the side chain is resonance stabilized. Complete the description of the resonance hybrid in the diagram above by drawing two other resonance structures and the curved-arrow notation used to derive them. (You can draw just the relevant part of the side chain.) Show all charges and unshared pairs!
a. The cation in the side chain is resonance stabilized. Complete the description of the resonance hybrid in the diagram above by drawing two other resonance structures and the curved-arrow notation used to derive them. (You can draw just the relevant part of the side chain.) Show all charges and unshared pairs!
b. Select the one true statement:
(1) Resonance makes this form of the amino acid a stronger base than it would be in the absence of such resonance.
(2) Resonance makes this form of the amino acid a weaker base than it would be in the absence of such resonance.
(3) Resonance makes this form of the amino acid a weaker acid than it would be in the absence of such resonance.
(4) Resonance makes this form of the amino acid a stronger acid than it would be in the absence of such resonance.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Aspartic acid is a naturally occurring amino
Q2: The configuration of all the naturally
Q3: Below are the structure and individual functional
Q4: Which, if any, of the structures represents
Q5: Fill in the wedge and dash bond
Q7: A tripeptide is shown below. (a) Give
Q8: The pKa values for glutamic acid are
Q9: A student wishes to use ion-exchange chromatography
Q10: A tripeptide is shown below. Label the
Q11: Show how the acetamidomalonate method can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents