Write an arrow-pushing mechanism for the isomerization of enol to cyclohexanone under (a) aqueous acidic conditions and (b) aqueous basic conditions. Show all proton transfer steps in each mechanism and two resonance structures for each intermediate.
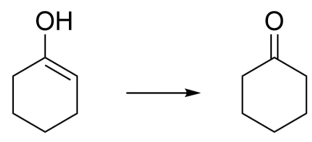
Correct Answer:
Verified
Q1: Give both enolates formed from deprotonation of
Q3: Choose the reaction conditions that would convert
Q4: When allowed to react with D2O in
Q5: Predict the major organic product for the
Q6: Indicate the missing starting material and product,
Q7: Deduce the structure of the starting material
Q8: Deduce the structure of the starting material
Q9: Choose the best reagent(s) for carrying out
Q10: One equivalent of ethanethiol reacts with a
Q11: Predict the major organic product for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents