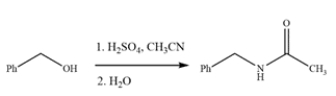
The Ritter reaction is a useful method for producing substituted amides. Provide a detailed, arrow-pushing mechanism for the transformation. Show all reactive intermediates and all proton transfer steps. (It is not necessary to show every resonance structure for intermediates.)
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Draw a structure for these carboxylic acid
Q2: Rank the priority of principal groups in
Q3: Draw the major organic product of the
Q4: Provide a detailed, arrow-pushing mechanism for the
Q6: Choose the best reagent(s) for carrying out
Q7: Describe the equilibrium constant for the reaction.
Q8: Propose a synthesis of the transformation.
Q9: Which reagents could be used to synthesize
Q10: Predict the major organic product of the
Q11: Predict the major organic product of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents