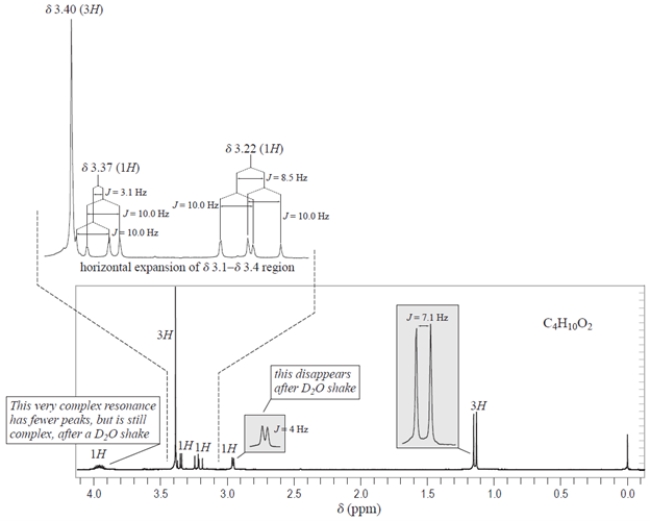
A compound X with the formula C4H10O2 has the NMR spectrum shown. The integrals are given over their respective resonances, and the J values are coupling constants. Deduce the structure of compound X and explain how you came to this conclusion. Hints: (1) The only chemical shift information you need is that protons on carbons to an oxygen have chemical shifts in the 3.2- 4.0 range. (2) You do not need to interpret the complex splittings at 3.22, 3.37, and 3.9 to deduce the structure, but you will need to interpret the splittings in the gray boxes.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q17: How many signals would you expect in
Q18: Explain how you could use proton decoupled
Q19: Deduce the structure of a compound
Q20: Deduce the structure of an unknown
Q21: a. Identify compound X with the formula
Q22: Deduce the structure of the unknown
Q24: Deduce the structure of a compound C6H14O
Q25: The NMR spectrum of the missing compound
Q26: The spectra were recorded for a
Q27: An unknown compound has a molecular formula
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents