Given the following information: 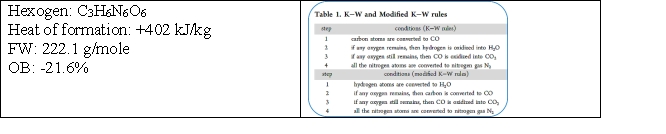 Predict the reaction products using the appropriate rules. How many moles of CO2 are produced in an explosion type reaction of 1 mole of hexogen?
Predict the reaction products using the appropriate rules. How many moles of CO2 are produced in an explosion type reaction of 1 mole of hexogen?
A) 1
B) 2
C) 3
D) 4
E) none of the above
Correct Answer:
Verified
Q1: What is the difference between a detonation
Q2: The ability of an explosive to cause
Q3: Pull-poppers are small devices that detonate with
Q4: The explosive ethylenedinitramine has a formula weight
Q5: Mercury fulminate is a shock-sensitive primary explosive
Q7: Given the following information: Q8: Why can't atmospheric oxygen be considered when Q9: What is the order of explosives built Q10: Why is a pipe bomb that is![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents