Based only on the illustration below it could be predicted that ice floats on liquid water because 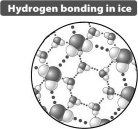
A) the crystal structure of ice is more regular than that seen in liquid water.
B) the distance between water molecules in ice is greater than in liquid water.
C) the cool temperature of ice reduces the extent of molecular motion relative to liquid water.
D) when ice forms the hydrogen bond in the water molecule becomes nonpolar; ice behaves like oil.
Correct Answer:
Verified
Q25: A molecule with the general formula CH₂O
Q28: One of the symptoms of kidney disease
Q29: Pure water has a pH of 7.
Q29: The sugar glucose has an important role
A)
Q30: The carbon dioxide you breathe out is
Q30: A solution with a pH of 3
Q31: An acid is a polar substance that
Q33: Which of the following levels of protein
Q36: The chemical reaction that represents the combustion
Q40: A solution with a pH of _
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents