It is possible to predict the amount of energy released during a chemical reaction (as shown in the illustration below) because the _____ law of thermodynamics states that _____. 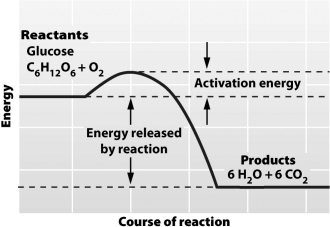
A) first; energy and matter are equivalent
B) second; any use of energy affects the entire universe
C) first; the total amount of energy in a defined system remains constant
D) second; energetic systems become less organized over time
Correct Answer:
Verified
Q7: The reuse of the same carbon molecules
Q8: During photosynthesis, light energy is converted into
Q9: Organisms that maintain a constant body temperature
Q10: Imagine a system consisting of a mousetrap
Q10: In accordance with the first law of
Q11: The term thermal energy describes the
A)orderly movement
Q13: Why is heat shown flowing from the
Q15: The second law of thermodynamics states that
A)metabolic
Q16: Plants and animals use different energy storage
Q17: _ reactions use energy to build complex
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents