The following is an energy diagram for the conversion of A ? B ?C. The energies of activation and DH's for each step are also given. Calculate DH overall as shown on the energy diagram for A ? B ? C.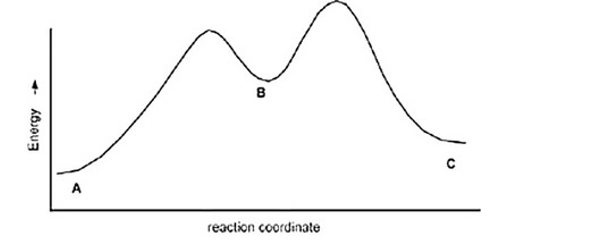
Ea (A ?B) =+10 Kcal
Ea (B?C) =+4 Kcal
DH (A ?B) =+8 Kcal
DH (B ?C) =-5 Kcal
A) +9 kcal
B) +7 kcal
C) None of these
D) +3 Kcal
Correct Answer:
Verified
Q6: Which of the following statements about enzymes
Q7: Calculate Ea for the conversion of C
Q8: Which of the following statements about substitution
Q9: How many steps are there in a
Q10: Which of the following statements is true?
A)
Q11: The conversion of acetyl chloride to methyl
Q13: Which of the following statements about equilibrium
Q14: A reaction that results in the formation
Q15: If the conversion of A to B
Q16: Which of the following statements about bond
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents