The stereoselective introduction of the C15 OH group on the w side chain of prostaglandins has presented a challenge for synthetic chemists. Recently, Mulzer and co-workers demonstrated a new route in which the w side chain is synthesized independently and then attached to the bicyclic counterpart. What is the appropriate sequence of reagents for the following synthesis? 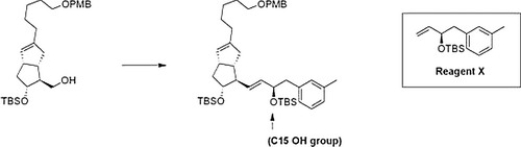
A) (1) PBr3; (2) Grubbs' catalyst, Reagent X; (3) KOC(CH3) 3
B) (1) TsCl, pyridine; (2) Grubbs' catalyst, Reagent X; (3) KOC(CH3) 3
C) (1) PBr3; (2) Ph3P=CH2; (3) Grubbs' catalyst, Reagent X
D) (1) PCC; (2) Ph3P=CH2; (3) Grubbs' catalyst, Reagent X
Correct Answer:
Verified
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents