Why would the alcohol in the following compound need to be protected before reaction? 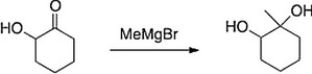
A) There is no need to protect the alcohol.
B) Magnesium is Lewis acidic and will coordinate with the alcohol.
C) The Grignard reagent will react with the alcohol before the ketone.
D) If it isn't protected, the product will be a carboxylic acid.
Correct Answer:
Verified
Q4: What is the product of the following
Q5: If the starting material has no stereogenic
Q6: What is the product of the following
Q7: What is the missing reagent in the
Q8: What is the product of the following
Q9: What is the product of the following
Q10: Both LiAlH4 and NaBH4 are reducing agents.
Q11: What is the starting material in the
Q12: A carbonyl group, C=O, and an alkene,
Q14: What is the missing reagent in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents