The structural formula for erythrose is drawn here. The carbon atoms are numbered from the carbonyl group. 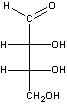
A) This is the only formula for erythrose; there are no stereoisomers.
B) This is both a D and an L structure, as determined by carbons 1 and 2.
C) This is D-erythrose, as determined by carbon 3.
D) This is L-erythrose, as determined by carbon 4.
Correct Answer:
Verified
Q7: Which of the following is an L
Q8: Which of the following molecules contain a
Q9: Which of the following molecules contain a
Q10: How many chiral carbons are present in
Q11: The structure of the compound 2,3,4-trihydroxybutanal is
Q13: How many stereoisomers would a carbohydrate with
Q14: What is the relationship of these compounds
Q15: What can be said about the relationship
Q16: Which of the following is a deoxy
Q17: Which of the following is an alcohol
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents