Phenol and toluene (structures shown below) have similar molecular weights.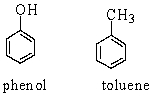 Which of the following statements accounts for the higher boiling point of phenol (182 C) than that of toluene (111 C) ?
Which of the following statements accounts for the higher boiling point of phenol (182 C) than that of toluene (111 C) ?
A) Toluene is more soluble in water than phenol.
B) Phenol molecules are primarily attracted to one another by London forces.
C) Toluene has greater hydrogen bonding interactions than phenol.
D) Phenol molecules are primarily attracted to one another by hydrogen bonds.
Correct Answer:
Verified
Q20: What is the IUPAC name of the
Q21: Provide the IUPAC name for the compound
Q22: The molecule responsible for the hot taste
Q23: The carboxylic acids share which characteristic with
Q24: The IUPAC name of the molecule below
Q26: The carboxylic acids with less than 11
Q27: Which of the following produce acidic solutions
Q28: Which of the following lowers the pH
Q29: The chapter refers to physiological pH, which
Q30: Phenols are organic acids, as are carboxylic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents