The equilibrium constant, Keq, for the ammonia synthesis below is found to be 6.0 x 10-2 at 500 C. Which of the following statements is true at equilibrium? 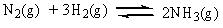
A) Product concentration is greater than reactant concentration.
B) Reactant concentration is greater than product concentration.
C) Reactant concentration is the same as product concentration.
D) Relative concentrations of reactants and products cannot be predicted.
Correct Answer:
Verified
Q13: Which of the following is the correct
Q14: Whenever an equilibrium constant, Keq, has a
Q15: When a reaction is at equilibrium,
A) it
Q16: Choose the equilibrium constant that indicates the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents