Kw, the equilibrium constant for the ionization of water by the equation below, is 1.0 x 10-14. What does that mean when we are considering pure water? 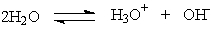
A) More ions exist than water molecules.
B) The majority of the molecules present are in the form of H2O.
C) The amount of water is the same as the amount of the ions present.
D) There will always be more hydronium ions present than water at equilibrium.
Correct Answer:
Verified
Q24: Which of the conditions below would drive
Q25: Which of the following statements is true?
A)
Q26: In the reaction below: Q27: What is the concentration of [H3O+] in Q28: Calculate the [OH-] in an aqueous solution Q30: A solution in which the concentration of Q31: Basic solutions have an H+ concentration Q32: Acidic solutions have an H+ concentration Q33: One liter of a solution is found Q34: When one is referring to pH, what![]()
A) greater
A) greater
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents