If 5.5 moles of pentane are burned according to the following equation: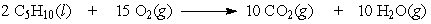 how many moles of oxygen are required?
how many moles of oxygen are required?
A) 41
B) 83
C) 11
D) 5.5
Correct Answer:
Verified
Q32: Which reaction is NOT an oxidation-reduction reaction?
A)
Q33: Ethyne, C2H2, used in welding react with
Q34: Isooctane, C8H18, a component of gasoline is
Q35: In the following reaction, the aldehyde has
Q36: In the following reaction, 10.0 moles of
Q38: According to the following equation, what mass
Q39: Methanol burns in air according to the
Q40: Balance the equation below and determine the
Q41: How many grams of oxygen (O2) are
Q42: In the following reaction, how many grams
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents