Which Atoms in the Following Molecule Would Have - charge Based on the Electronegativities of the Elements
Which atoms in the following molecule would have - charge based on the electronegativities of the elements? 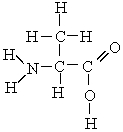
A) H and O
B) O and C
C) C and N
D) N and O
Correct Answer:
Verified
Q13: Which molecule is nonpolar even though it
Q14: Which of the following bonds can be
Q15: Which bond is most polar?
A) B-Cl
B) C-Cl
C)
Q16: Oxygen is more electronegative than carbon, so
Q17: In which bond does the oxygen atom
Q19: Which atoms in the following molecule
Q20: Which bond is least polar?
A) H-F
B) H-Cl
C)
Q21: Which molecule has a linear geometry?
A) SO2
B)
Q22: Which of the following molecules is nonpolar?
A)
Q23: Which of the following molecules is polar?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents