The strongest noncovalent interaction that can occur between two methyl alcohol molecules is ___. 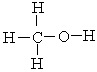
A) dipole-dipole forces
B) hydrogen bonding
C) ion-dipole interaction
D) London force
Correct Answer:
Verified
Q21: Which molecule has a linear geometry?
A) SO2
B)
Q22: Which of the following molecules is nonpolar?
A)
Q23: Which of the following molecules is polar?
A)
Q24: What is the shape around C atom
Q25: The strongest noncovalent interaction that can occur
Q27: Organic compound must contain which of the
Q28: The following molecule belongs to which family
Q29: Which pairs of molecules can form a
Q30: Serotonin is a molecule involved in the
Q31: The following molecule belongs to which organic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents