The kinds of interactions that exist between CH3COOH molecules include dipole-dipole (including hydrogen bonding) interactions and London forces.
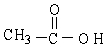
Correct Answer:
Verified
Q32: The CaF bond in CaF2 is nonpolar.
Q33: Potassium has lower electronegativity than F.
Q34: CH2Cl2 is polar.
Q35: The Si-Si bond in Cl3SiSiCl3 is expected
Q36: Dioxygen difluoride whose actual three dimensional shape
Q38: Hydrocarbons can take part in dipole-dipole interactions.
Q39: The molecule shown below contains an aldehyde
Q40: Ketone is present in Vanillin.

Q41: The shape of H2O is bent.
Q42: Of the two elements, lithium and chromium,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents