A 1.0 x 102- gram sample is found to be pure alanine, an amino acid found in proteins. How many moles of alanine are in the sample? 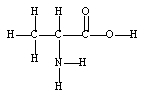
A) 0.20 moles
B) 0.90 moles
C) 1.1 moles
D) 890 moles
Correct Answer:
Verified
Q41: Aluminum sulfate, Al2(SO4)3, can be used in
Q42: What is the mass in grams of
Q43: Calculate the mass of 10.00 moles of
Q44: Glycerol, often called glycerine, was used as
Q45: Ethanol, C2H5OH, is found in alcoholic beverages.
Q47: What is the mass of 0.00142 mole
Q48: How many molecules of aspirin (C9H8O4) are
Q49: Ag+ is an ion with 47 protons
Q50: A Roman numeral is used in the
Q51: Helium forms an ion with a 2+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents