One of the compounds listed is an organic base that functions as a proton acceptor. Identify this organic base.
A) CH3-CH2-CH2-O-H
B) 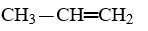
C) 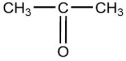
D) 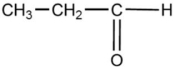
E) CH3-CH2-NH2
Correct Answer:
Verified
Q63: Which compound is least soluble in water?
A)
Q64: Which compound is a Brønsted base?
A)
Q65: Which one of the species below is
Q66: Which compound is insoluble in water?
A)
Q67: A functional group containing nitrogen is found
Q69: The compound, trimethylamine is a(n)_ and has
Q70: Dodecylamine, CH3(CH2)10CH2NH2, is insoluble in water. Yet,
Q71: Octylamine, CH3(CH2)7NH2, is insoluble in water. Yet,
Q72: The compound below has which functional groups?
Q73: The compound below has which functional groups?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents