The only stable isotope of fluorine is 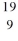 F. What type of radioactivity would you expect from the unstable isotope
F. What type of radioactivity would you expect from the unstable isotope 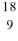 F in order to form a more stable product? Hint: the goal is not to form fluorine-19
F in order to form a more stable product? Hint: the goal is not to form fluorine-19
Correct Answer:
Verified
Q105: Tritium is an isotope of hydrogen that
Q106: The emission of a positron from a
Q107: When high energy radiation passes through gaseous
Q108: Nuclear fission is a process by which
Q109: Fission reactions used for generation of electrical
Q110: The waste from nuclear power plants is
Q111: Nuclear power plants use much a much
Q113: The only stable isotope of iodine is
Q114: Radium-226, which undergoes a first order nuclear
Q115: Why are samples of uranium found in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents