Using the standard reduction potentials: 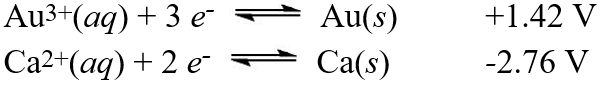 Calculate the value of E°cell for the reaction:
Calculate the value of E°cell for the reaction:
2Au(s) + 3Ca2+(aq) 2Au3+(aq) + 3Ca(s)
A) -1.43 V
B) +1.34 V
C) -4.18 V
D) +4.18 V
E) -1.34 V
Correct Answer:
Verified
Q12: The electrode for which the standard reduction
Q13: Using these metal ion/metal standard reduction
Q14: Using these metal ion/metal standard reduction
Q15: Using these metal ion/metal standard reduction
Q16: Using these metal ion/metal reaction potentials:
Q18: Using the standard reduction potentials::
Q19: For the reaction, 2 Cr2+(aq)+ Cl2(g)
Q20: The cell described by the net reaction:2U(s)+
Q21: Consider this electrochemical cell:Pt | Pu3+(aq),
Q22: The cell described by the reaction,2 Co3+(aq)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents