Using these metal ion/metal standard reduction potentials 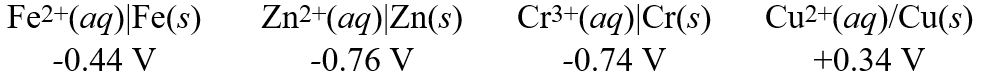 A galvanic cell is composed of these two half-cells:
A galvanic cell is composed of these two half-cells:
Cr3+(aq)| Cr(s)
Cu2+(aq)| Cu(s)
What is the standard reduction potential for the cell reaction of this galvanic cell?
Correct Answer:
Verified
Q108: The Faraday constant is equal to the
Q109: What is the purpose of an electrolyte
Q110: Given the following notation for an electrochemical
Q111: Sketch a galvanic cell with metallic zinc
Q112: Consider an electrochemical cell based on the
Q114: If the measured voltage of the cell
Q115: Consider the following reaction: 2Fe2+(aq)+ Cu2+ →
Q116: Consider the following reaction: 2Cu+(aq)+ Ni2+ →
Q117: In the lead storage battery, the electrolyte
Q118: In lithium ion batteries, lithium ions are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents