The normal melting point of benzoic acid is 122.4°C. Predict the signs of H, S, and ?G for the process in which liquid benzoic acid freezes at 120°C and 1 atm: C7H6O2(l) .C7H6O2(s) 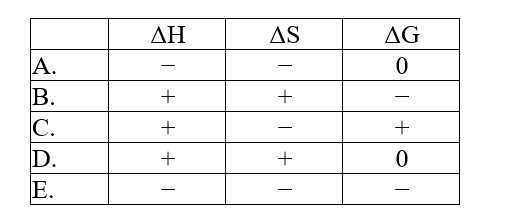
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q23: Which set below has the species listed
Q24: Which set below has the species listed
Q25: For a certain chemical reaction,
Q26: For a certain chemical reaction,
Q27: The requirement for a spontaneous chemical
Q29: The normal melting point of carbon
Q30: The normal melting point of naphthalene
Q31: Which property associated with a chemical
Q32: A negative sign for
Q33: For the reaction 2NO(g)+ O2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents