Short Answer
Calculate ΔG° at 25°C for the reaction 2HNO3(l)+ NO(g)→ 3NO2(g)+ H2O(l). 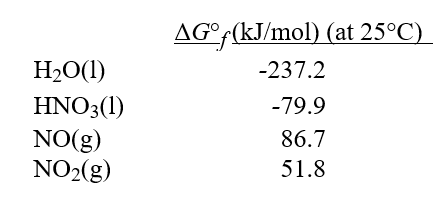
Correct Answer:
Verified
Related Questions
Q77: Consider a reaction that is both endothermic
Q78: Describe, using the free energy relationship ΔG
Q79: HI has a normal boiling point of
Q80: The entropy of perfectly ordered pure crystalline
Q81: Using the data,
I2(g), ΔH°f =
Q83: Ammonia (NH3)has a normal boiling point of
Q84: For a chemical reaction, ΔH° = +21.16
Q85: For a chemical reaction, ΔH° = +31.16
Q86: For the system, 2 NO2(g) 
Q87: For the system, 2 NO2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents