Given the following information: 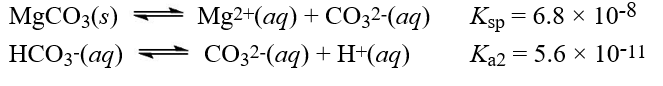 What is the equilibrium constant for the reaction,MgCO3(s)+ H+(aq)
What is the equilibrium constant for the reaction,MgCO3(s)+ H+(aq)  Mg2+(aq)+ HCO3-(aq)
Mg2+(aq)+ HCO3-(aq)
Correct Answer:
Verified
Q86: Calculate the minimum concentration of Cr3+ that
Q87: Calculate the concentration of chloride ions in
Q88: A student mixes 50.00 mL of 0.500
Q89: The solubility of lead(II)iodide is 0.064 g/100
Q90: 200 mL of an aqueous solution contains
Q92: For the H2CO3/ HCO3/CO32- system at room
Q93: Many metal sulfides have _ solubilities.
Q94: Metal sulfides actually dissolve in water by
Q95: Metal cations in the chloride solubility group
Q96: Group II and group III cations (acid-insoluble
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents