The sulfide ion can react with the sulfur trioxide molecule to produce a thiosulfate ion, as shown below. 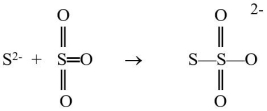 Which reactant is functioning as the Lewis base?
Which reactant is functioning as the Lewis base?
Correct Answer:
Verified
Q76: Three oxoacids with the formulas shown are
Q77: Three binary acids with the formulas shown
Q78: Which one of the two species, H2Se
Q79: Which one of the two species, HF(aq)or
Q80: Which one of the two species, H2S
Q82: Arrange the acids H2Se, H2Te, and H2S
Q83: Selenium atoms can react with the sulfite
Q84: The oxide ion can react with the
Q85: Explain how NH3 can be both a
Q86: Which solution is more acidic, AlCl3(aq)or InCl3(aq)?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents