The following reactions have equilibrium values all measured at 500 K. Arrange them in order of increasing tendency to proceed to completion (least completion ? greatest completion) . 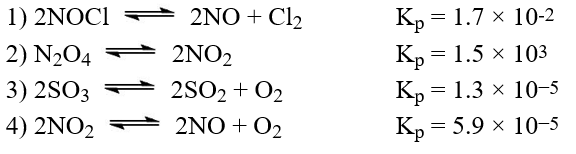
A) 2 < 1 < 3 < 4
B) 4 < 3 < 1 < 2
C) 3 < 1 < 4 < 2
D) 3 < 4 < 1 < 2
E) 4 < 3 < 2 < 1
Correct Answer:
Verified
Q24: For the chemical reaction, N2(g)+ 3H2(g)
Q25: The equilibrium constant for the reaction, R2
Q26: The following reactions occur at 298 K.
Q27: The following reactions occur at 298 K.
Q28: For a system, H2(g)+ I2(g) 
Q30: For the reaction, 2SO2(g)+ O2(g) 
Q31: For the reaction, 2SO2(g)+ O2(g) 
Q32: For the reaction, 2SO2(g)+ O2(g) 
Q33: Given the reaction, 2NO(g)+ O2(g) 
Q34: The reaction, 2 SO3(g) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents