The following reactions occur at 500 K. Arrange them in order of increasing tendency to proceed to completion (least → greatest tendency). 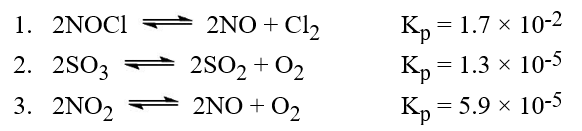
Correct Answer:
Verified
Q79: Write the equilibrium law for the following
Q80: Write the equilibrium law for the following
Q81: Write the equilibrium law for the following
Q82: Write the equilibrium law for the following
Q83: Write the equilibrium law for the following
Q85: The following reactions occur at 500 K.
Q86: For the reaction SO2Cl2(g) Q87: For the reaction SO2Cl2(g) Q88: For the reaction SO2Cl2(g) Q89: For the reaction 3C(s)+ 3 H2(g) Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

