The reaction mechanism proposed for the decomposition of H2O2 is 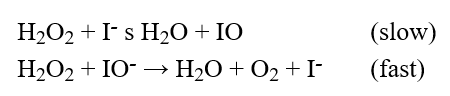 Which statement is true?
Which statement is true?
A) The reaction is second order with respect to I-.
B) I is an intermediate.
C) The reaction is first order with respect to I-.
D) IO- is a catalyst.
E) The reaction is zero order with respect to I-.
Correct Answer:
Verified
Q84: For a particular chemical reaction, the rate
Q85: The activation energy for a reaction can
Q86: The reaction: 2A + 2B
Q87: Which statement about the slow step in
Q88: If the reaction H2(g)+ Cl2(g)
Q90: The reaction mechanism proposed for the decomposition
Q91: Suppose the reaction A + B
Q92: The reaction: A + 3B
Q93: The reaction: A + 3B
Q94: A variable which has no effect on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents