The decomposition of hydrogen peroxide in solution was studied in the laboratory, and the following mechanism was proposed based on the experimental data. 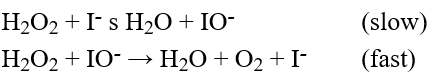 Which one of the following statements is false?
Which one of the following statements is false?
A) The reaction is first order with respect to H2O2.
B) The reaction is first order with respect to I-.
C) I- is a catalyst.
D) IO- is an intermediate.
E) The reaction is first order with respect to IO-.
Correct Answer:
Verified
Q141: The step in a multistep reaction mechanism
Q142: A catalyst that lowers the activation energy
Q143: The reaction rates for similar reactants are
Q144: Given this data from a study on
Q145: The reaction A + 2B → products
Q146: A nuclear transmutation reaction is a first-order
Q147: The reaction, 2 NO2(g)→ 2 NO(g)+ O2(g),
Q148: The half-life of a first order chemical
Q149: Suppose the reaction, 2A + 2B
Q151: Many reactions require that the initial reactant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents