Arrange these aqueous solutions in order of increasing boiling points: 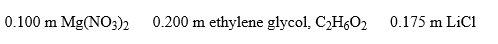
A) C2H6O2 < Mg(NO3) 2 < LiCl
B) Mg(NO3) 2 < LiCl < C2H6O2
C) C2H6O2 < LiCl < Mg(NO3) 2
D) LiCl < C2H6O2 < Mg(NO3) 2
E) Mg(NO3) 2 < C2H6O2 < LiCl
Correct Answer:
Verified
Q41: During osmosis,
A)pure solvent passes through a membrane
Q42: A very dilute solution contains 116 mg
Q43: Which aqueous solution will have the highest
Q44: Which aqueous solution will have the lowest
Q45: Which solution has the highest osmotic pressure
Q47: A dilute aqueous solution of CaCl2 contains
Q48: At 28.0°C, the vapor pressure of n-propyl
Q49: A molecular solute with a molar
Q50: A solution contains 221 g of glycerol
Q51: A solution, which was made by dissolving
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents