A solution of chloroform (CHCl3)and acetone ((CH3)2CO)exhibits a negative deviation from Raoult's law similar to that shown on the diagram below. What does this tell us about the solution with respect to it being an ideal solution and the strength of the interactions between acetone and chloroform? This result shows us that the solution is ________ ideal and the interactions between chloroform-chloroform and acetone-acetone are ________ than the interactions between chloroform/acetone. 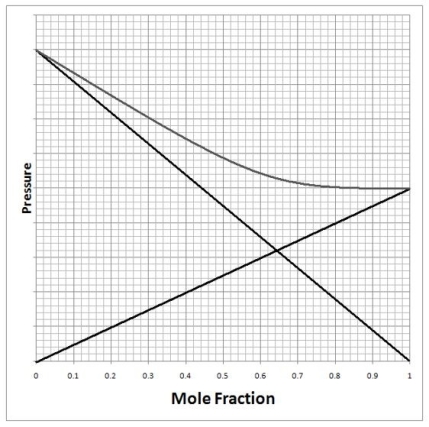
Correct Answer:
Verified
Q95: What is the mass percent of CdSO4
Q96: How many grams of water are needed
Q97: Calculate the molality of a 25.0% by
Q98: What is the mole fraction of ethylene
Q99: Consider a 0.80 M Al(NO3)3 solution. What
Q101: Arrange the following solutions in order of
Q102: Calculate the freezing point of a solution
Q103: Describe what a colligative property is. Explain
Q104: Raoult's law expresses that the higher the
Q105: The van't Hoff factor describes to what
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents