The following questions refer to the basic phase diagram below with gas, liquid, and solid phases present and labeled. 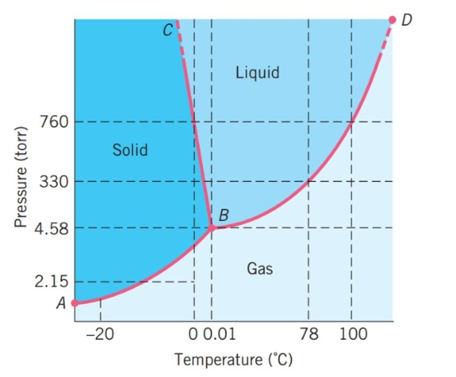
-If the substance starts at a pressure of 330 torr and -20°C and is heated at constant pressure to 20°C, what phase transition will occur?
Correct Answer:
Verified
Q139: The heating of a liquid above its
Q140: The heat capacity of liquid water is
Q141: Substances with higher vapor pressures at room
Q142: Which of the following substances has the
Q143: A solid chemical substance whose triple point
Q145: The following questions refer to the basic
Q146: The following questions refer to the basic
Q147: The following questions refer to the basic
Q148: The following questions refer to the basic
Q149: The following questions refer to the basic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents