Keshon has an equation that gives the relationship between the air pressure at sea level, p0, and the air pressure, p, at a height, h (in meters), above sea level:h = 4405 ln 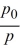 He found a value for ΔΗvap of water, 41,108 J mol-1. Keshon has now challenged two other whiz kids in the class with this problem: Their weather app has given the barometric pressure at sea level as 30.18 inches of mercury. What is the temperature of the boiling water in a teapot at a mountain camp which is 2200 meters (about 7220 feet)above sea level?Hint: Use the Clausius-Clapeyron equation and pay attention to units.
He found a value for ΔΗvap of water, 41,108 J mol-1. Keshon has now challenged two other whiz kids in the class with this problem: Their weather app has given the barometric pressure at sea level as 30.18 inches of mercury. What is the temperature of the boiling water in a teapot at a mountain camp which is 2200 meters (about 7220 feet)above sea level?Hint: Use the Clausius-Clapeyron equation and pay attention to units.
Correct Answer:
Verified
Q179: During a rain storm, the surface of
Q180: Why would the coffee purchased in a
Q181: Why does CH3-CH2-O-H have a higher boiling
Q182: Explain how you could cause pure water
Q183: A substance has a melting point of
Q184: A substance has a melting point of
Q185: A substance has a normal boiling point
Q186: Based on the phase diagram of water,
Q188: Broken laboratory glass can sometimes be repaired
Q189: Draw the unit cell for the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents