Consider the cyclobutadiene structure shown below. How many bonds are there? 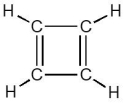
A) 1
B) 2
C) 3
D) 4
E) 8
Correct Answer:
Verified
Q84: An energy level scheme for the
Q85: An energy level scheme for the
Q86: An energy level scheme for the
Q87: An energy level scheme for the
Q88: According to MO theory, the diatomic C2
Q90: What is the total number of
Q91: The electrons in the delocalized molecular orbitals
Q92: Which species from the list, NO3, NO2,
Q93: The energy separation between the nearest empty
Q94: In solid sodium metal, which orbitals contribute
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents