Consider a hypothetical species with an octahedral geometry shown below, with three different placements of its bonded atoms (indicated as "A") and lone pairs (indicated as blank) . 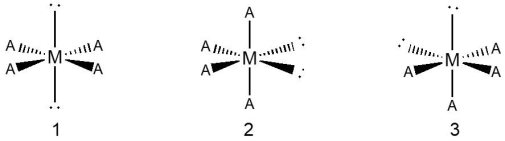 Which species, 1, 2, or 3, is the preferred orientation of atoms and lone pairs?
Which species, 1, 2, or 3, is the preferred orientation of atoms and lone pairs?
A) 1
B) 2
C) 3
D) 2 and 3 are equally probable.
E) None of these arrangements of atoms and lone pairs is possible.
Correct Answer:
Verified
Q186: The P-P single bond is stronger than
Q187: Electronic devices which are based on graphene
Q188: Graphene are sheet-like layers made up of
Q189: In graphene, carbons has sp2 hybrid orbitals.
Q190: One way to make graphene is to
Q191: The number of nonbonding electron domains in
Q192: The total number of electron domains in
Q194: A molecule has a measurable dipole moment.
Q195: Kevin, the high school whiz kid, is
Q196: Which molecular structure is most likely
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents